In 2018, the Compressed Gas Association celebrated a number of regulatory successes, including the adoption of CGA recommended practices for GHS-compliant labeling, design aspects of cylinder and valve packages, requalification of manifolded cylinders and several other issues. As we look forward to 2019, there are several key areas of regulatory activity to monitor for impacts to our industry. We will continue to collaborate with our members, partner organizations like GAWDA, and our regulatory stakeholders to promote acceptance of industry positions on these issues and others that come up throughout the year.
Consumer Safety
As our industry’s products become more common in the consumer landscape — in beverages, food presentations, spa therapies and other applications that put them directly in contact with the public — we expect that there will be continued regulatory activity to ensure consumer safety. The industry saw a clear example of this activity in 2018 when the U.S. Food & Drug Administration issued an alert advising consumers to avoid eating, drinking or handling food products prepared with liquid nitrogen at the point of sale.
Reflecting this changing landscape, CGA’s board of directors approved a modification of our scope to include the development of basic safety considerations for end use. This will allow CGA to focus more on near consumer safety issues. In 2019, we plan to augment our safety materials with the development of additional safety posters, eLearning modules and other materials designed to communicate critical safety concepts to someone new to handling our industry’s products and related equipment. We will also continue to issue safety alerts to provide timely warnings about industry safety issues to the public.
Medical Gas Regulations
In 2018, the FDA held a series of public workshops to solicit stakeholder input for the development of separate regulations for medical gases. Separate regulations are needed to prevent our industry from being subjected to ineffective requirements intended for traditional pharmaceuticals. CGA and GAWDA worked together to develop and submit input to the FDA, provide verbal comments on behalf of the industry during the public workshops and respond to FDA inquiries resulting from the workshops.
As a result of these public meetings, we anticipate that the FDA will propose revisions to certain medical gas regulations in the second quarter of 2019. These proposed revisions are anticipated to include specific changes to current good manufacturing practice (cGMP) regulations in order to align the cGMP with CGA positions on appropriate and effective regulations for medical gases. The regulatory revisions are also expected to address updated requirements for security and environmental conditions at medical gas wholesale facilities, changes to general labeling requirements and clarifications to post-market reporting regulation applicability to medical gases. Acceptance of the CGA/GAWDA proposals will result in more appropriate and effective medical gas regulations and less controversy during FDA and state agency inspections with increased patient and public safety.
Unique Device Identifier Requirements
In September 2013, the FDA published a final rule instituting unique device identifier (UDI) requirements for medical devices. As originally written, the UDI requirements extended to a number of products in our industry such as calibration gases, laser gases, pressure regulators, cylinders, valves, gas pressure transducers, hoses, yokes, flowmeters and more. CGA and GAWDA have reached out to the FDA numerous times since these rules were introduced to request that our industry’s Class 1 and Unclassified products be exempt from these requirements on the basis that they represent a low risk to patient safety.
Although the final UDI compliance phase does not become effective until 2020, CGA and GAWDA are collaborating to provide comments on the FDA’s interpretation of what medical devices are subject to these regulations. We have requested meetings with the FDA to clarify that plain cylinders and cylinders with plain valves are not medical devices, and should not be subject to UDI requirements. Without this clarification, many in the industry will be subject to unnecessary marking and tracking requirements. CGA and GAWDA are also seeking to resolve confusion around the application of UDI codes to valve integrated pressure regulators (VIPRs).
Updates to U.S. Hazardous Material Regulations
The UN Sub-Committee of Experts on the Transportation of Dangerous Goods (SCETDG) has completed their 2017–2018 biennium and is expected to publish the 21st revised edition of the UN Recommendations on the Transport of Dangerous Goods – Model Regulations (known as the UN Orange Book) in the first quarter of 2019. It has been the DOT’s practice to propose changes the U.S. Hazardous Material Regulations based on the revised Model Regulations, as well as on revisions to modal regulations such as International Civil Aviation Organization or International Maritime Dangerous Goods codes.
Changes to the UN Model Regulations that will impact our industry include:
- Adoption of several ISO standards on the inspection and testing of various cylinders, containers and pressure drums.
- Change of the LC50 values for various toxic gases, which could affect their transport classifications.
- Prohibit the manufacture and use of two-part composite cylinders and tubes but without liners, such as certain two-part propane cylinders.
- Adoption of the 2017 edition of ISO 10156, Gas cylinders – Gases and gas mixtures – Determination of fire potential and oxidizing ability for the selection of cylinder valve outlets, which establishes required valve outlet connections for flammable gas mixtures.
Updates to Hazard Communication Standards
The U.S. Occupational Safety and Health Administration is considering updating its Hazard Communication Standard in order to align it with the seventh edition of the UN’s Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The current OSHA Hazard Communication Standard references the third edition of the UN GHS. Aligning with the seventh revised edition will add pyrophoric gases and chemically unstable gases as separate entries under flammable gas and will require revisions to precautionary statements and some first aid statements. Aligning OSHA’s Hazard Communication Standard to the seventh edition will necessitate changes to CGA C-7 and cylinder GHS labels. A proposed rulemaking could be published this year.
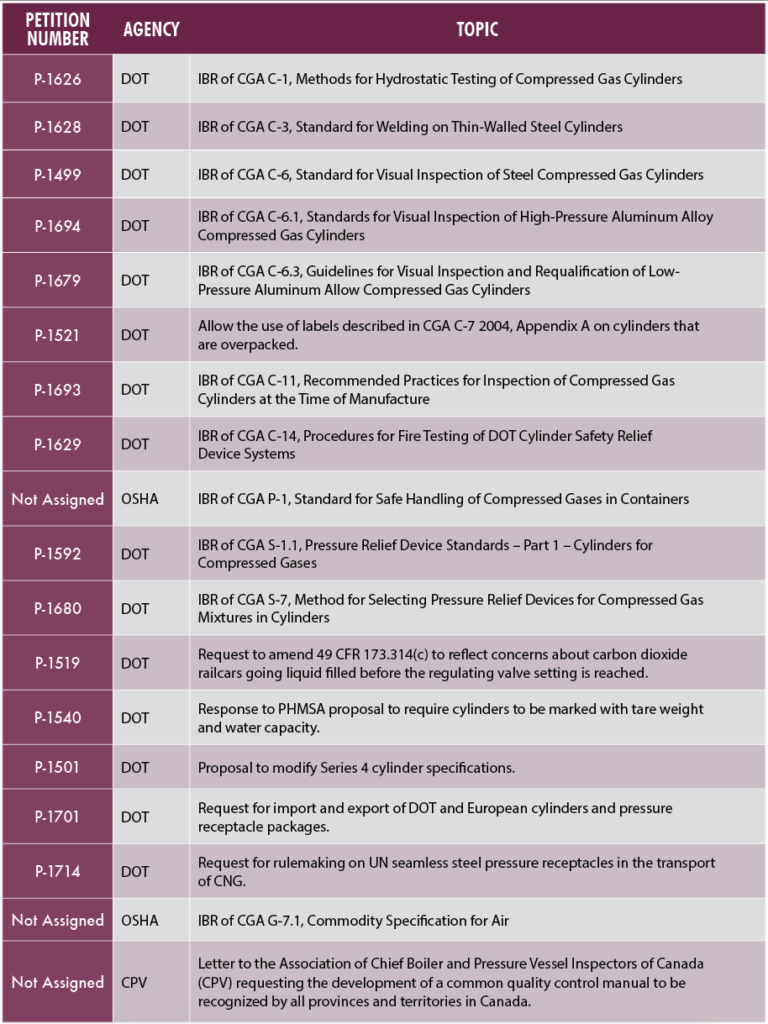
Regulatory Action on CGA Petitions
CGA is awaiting regulatory action on 18 previously submitted petitions and is actively working with our technical committees to develop additional petitions. These petitions request the incorporation by reference (IBR) of CGA publications or the adoption of industry positions on a specific issue. The table below summarizes our current pending petitions.
Bulk Medical Gas Supply Systems Certification
Recent changes to NFPA 55, Compressed Gases and Cryogenic Fluids Code, include a requirement for personnel who install and maintain bulk compressed medical gas (CMG) supply systems to be certified to CGA M-1, Standard for Medical Gas Supply Systems at Health Care Facilities. Previously, installers were required to be certified to ASSE 6015, Bulk Medical Gas Systems Installers, which defines training and competency requirements for technicians to be certified for bulk CMG supply system work but does not establish technical requirements for system design or maintenance. The adoption of CGA M-1 as a certification standard provides bulk CMG system installers with an alternative to ASSE 6015 that specifically addresses the unique considerations and hazards of the medical gases industry.
In 2018, the CGA Certification Board LLC (www.CGACB.org) launched a certification program to address this requirement. The CGA M-1/ASSE 6015 certification has been developed by subject matter experts with practical experience in designing, locating, installing, commissioning, maintaining, testing, removing and documenting work for bulk CMG supply systems. The exam covers all applicable standards, codes and regulations, including CGA M-1, ASSE 6015, NFPA 55, NFPA 99 and elements of the FDA regulations for medical gases found in Title 21 of the Code of Federal Regulations. The CGACB will be offering the CGA M-1/ASSE 6015 certification exam throughout the U.S. in 2019.








